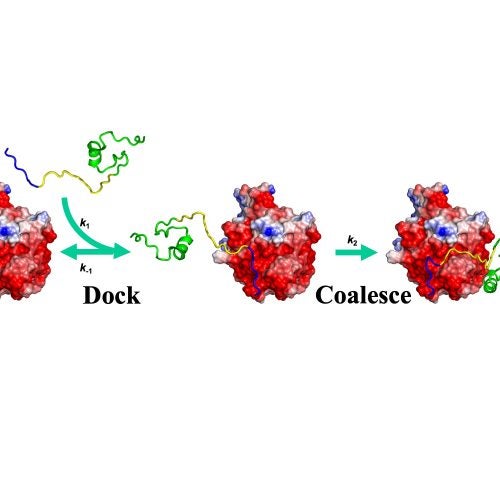
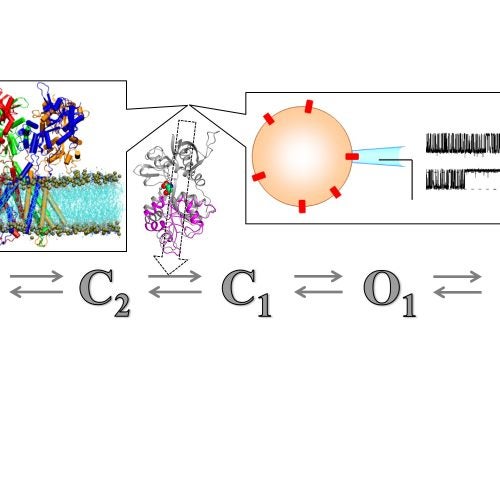
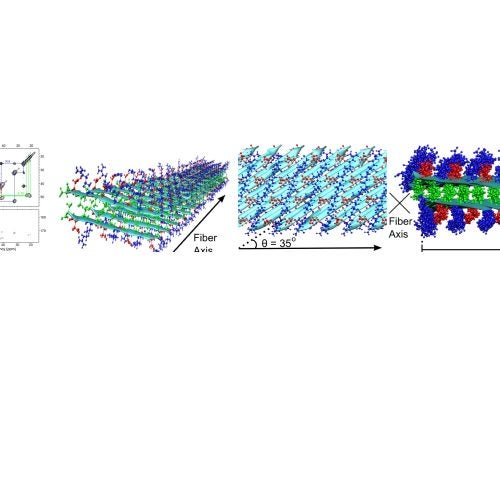
The Zhou group uses theory, computation, and experiment to address a range of topics in molecular and cellular biophysics. Four main areas are: (1) thermodynamic and dynamic properties of phase-separated biomolecular condensates; (2) membrane association and binding kinetics of intrinsically disordered proteins; (3) structures and pathways of the self-assemblies of amyloid-β and other amyloidogenic proteins; and (4) functional mechanisms of glutamate-receptor ion channels.
Research Overview Heading link
News and Notable Heading link
-
Hot Publication Our evaluation of AlphaFold as a landmark published in Faculty Reviews
-
Grants The Zhou group is supported by grants from the National Institutes of Health.
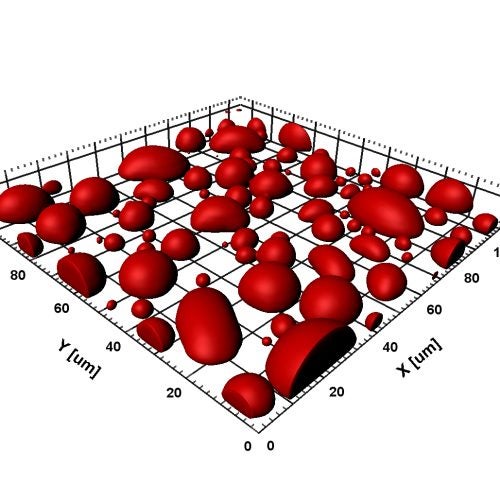
Falling and Fusion of Protein Droplets Heading link
Under gravity, protein droplets fall and then fuse on a glass slide. Macromolecular regulators can promote or suppress droplet formation as well as affect the material properties of the droplets. Read paper




