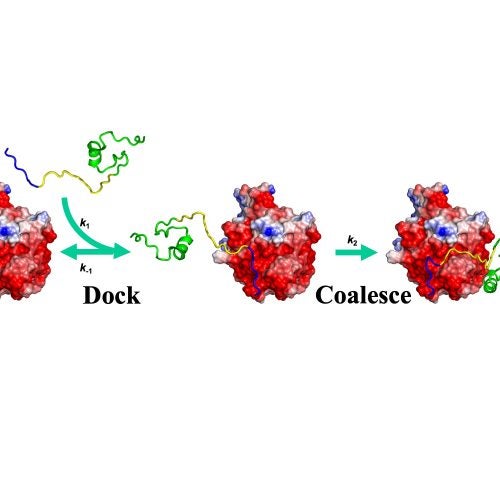
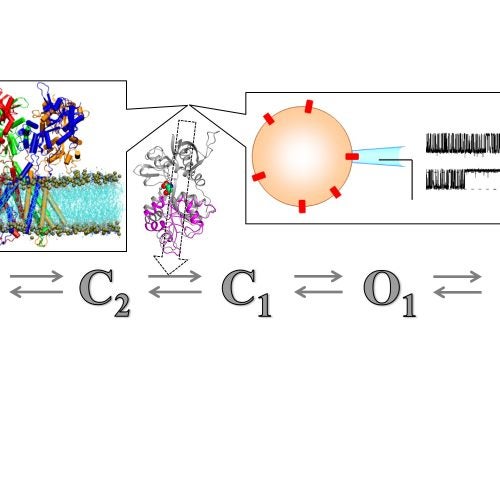
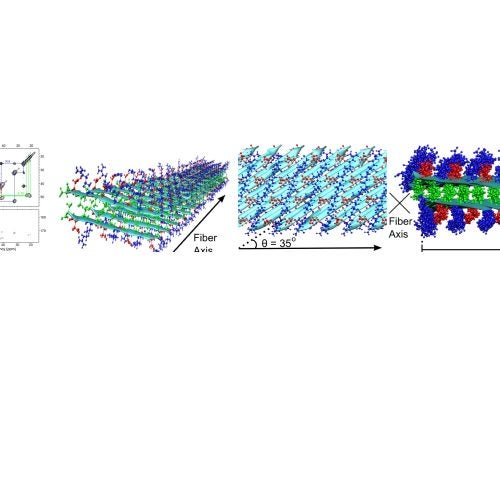
The Zhou group uses theory, computation, and experiment to address a range of topics in molecular and cellular biophysics. Four main areas are: (1) thermodynamic and dynamic properties of phase-separated biomolecular condensates; (2) membrane association and binding kinetics of intrinsically disordered proteins; (3) structures and pathways of the self-assemblies of amyloid-β and other amyloidogenic proteins; and (4) functional mechanisms of glutamate-receptor ion channels.
Research Overview
News and Notable
-
Hot Publication Our work reporting extreme DNA compaction by protamine published in JACS
-
Grants The Zhou group is supported by grants from the National Institutes of Health.
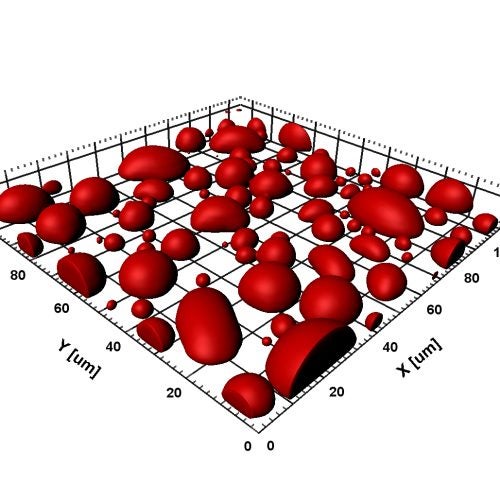
Falling and Fusion of Protein Droplets
Under gravity, protein droplets fall and then fuse on a glass slide. Macromolecular regulators can promote or suppress droplet formation as well as affect the material properties of the droplets. Read paper




